Important Questions/PYQs
One Mark Questions
1. Write chemical equation for the reaction of zinc metal on sodium hydroxide.
View AnswerAns. Zn (s) + 2NaOH (aq) — > Na2ZnO2 (aq) + H2 (g)
2. Three acidic solutions A, B and C have pH = 0, 3 and 5 respectively.
a) Which solution has the highest concentration of H+ ions?
b) Which solution has the lowest concentration of H+ ions?
View AnswerAns. a) The solution with pH = 0 has highest concentration of H+ ions
b) The solution with pH = 5 has lowest concentration of H+ ions.
3. What is meant by p and H in pH?
View AnswerAns. p stands for ‘potenz’ in German meaning power, H stands for hydrogen.
4. Define alkalies and give an example.
View AnswerAns. Water soluble bases are called alkalies e.g., NaOH
5. Mention the range of pH for identification of a base.
View AnswerAns. 7.1 to 14 is the pH range for bases.
6. How chloride of lime differs from calcium chloride?
View AnswerAns. CaOCl2 is the chloride of lime whereas CaCl2 is calcium chloride.
7. What is meant by water of crystallization in a substance?
View AnswerAns. The water molecules associated with crystalline solid are called water of crystallization.
8. Write the chemical name and chemical formula of washing soda.
View AnswerAns. Na2CO3.10H2O, sodium carbonate deca-hydrate is washing soda.
9. What effect does an increase in concentration of H+ (aq) ions in a solution have on pH of solution?
View AnswerAns. Increase in H+ concentration will lead to decrease in pH.
10. Name a gas evolved when dilute HCl reacts with sodium hydrogen carbonate. How is it recognised?
View AnswerAns. Carbon dioxide, it turns lime water milky. In this way CO2 gas is recognised.
11. What are olfactory indictors?
View AnswerAns. Those indicators whose smell changes in acidic and basic solutions.
12. Why does 1M HCl solution have a high concentration of H+ ions than 1 M CH3COOH solution?
View AnswerAns. It is because 1M HCl is a strong acid and it is completely ionized in aqueous solution whereas CH3COOH is a weak acid, so it is only partially ionized.
13. Write the chemical equation representing the action of atmospheric CO2 gas on bleaching powder when left exposed in open.
View AnswerAns. CaOCl2 + CO2 —- > C aCO3 + Cl2
14. How will you test for the gas which is liberated when hydrochloric acid reacts with an active metal?
View AnswerAns. Bring a burning matchstick near the gas. If it is burnt with a ‘pop’ sound, the gas is H2
15. How is the pH of solution of an acid influenced when it is diluted?
View AnswerAns. pH of the solution increases when it is diluted.
16. At what pH rain water is said to be acidic?
View AnswerAns. When pH < 5.5, the rain water becomes acidic.
17. Which gas is evolved when dilute hydrochloric acid reacts with zinc metal? Write the molecular formula of this gas.
View AnswerAns. Dihydrogen gas H2
18. Dry HCl gas does not change the colour of dry blue litmus. Give reason to justify it.
View AnswerAns. Dry HCl (g) does not form ions, therefore it does not affect dry blue litmus.
19. Why is HCl a stronger acid than acetic acid?
View AnswerAns. HCl is completely ionized in aqueous solution whereas acetic acid is only partially ionized in aqueous solution.
20. Name the chemicals used in acid fire extinguisher and the gas evolved from it when it is used?
View AnswerAns. NaHCO3 (Sodium hydrogen carbonate) and H2SO4 (Sulphuric acid). The gas evolved is carbon dioxide.
21. Which is stronger acid, with pH = 5 or with pH = 2?
View AnswerAns. The acid with pH = 2 is a stronger acid.
22. A compound which is prepared from gypsum has the property of hardening when mixed with a proper quantity of water. Identify the compound and write its chemical formula.
View AnswerAns. CaSO4. ½ H2O (Plaster of Paris), Calcium sulphate hemihydrate.
23. What is meant by term pH of solution? The pH of rain water collected from two cities ‘A’ and ‘B’ were found to be 6.0 and 5.0 respectively. The water of which city will be more acidic?
View AnswerAns. pH of solution is measure of Hydrogen (H+) ion concentration, which is, in turn, a measure of acidity. It determines the strength of acid and base. Rainwater with pH = 5 is more acidic.
24. A few drops of sulphuric acid are added to water before electrolysis, why?
View AnswerAns. Water is not a good conductor of electricity. Few drops of sulphuric acid makes it better conductor of electricity.
25. Write the names of two salts belonging to sodium family.
View AnswerAns. NaCl, Na2CO3 are two salts belonging to sodium family.
26. Which among distilled water, tap water and sea water is the best conductor of electricity?
View AnswerAns. Sea water is a better conductor due to the presence of ions.
27. Name the acids present in (i) nettle sting, (ii) curd
View AnswerAns. (i) Formic acid, HCOOH
(ii) Lactic acid, CH3-CH(OH)-COOH
28. Name a salt which does not contain water of crystallization.
View AnswerAns. NaHCO3 is a salt that does not contain water of crystallization.
29. Write the name and chemical formula of the product formed by heating gypsum at 373 K
View AnswerAns. Plaster of Paris, CaSO4. ½ H2O
30. The pH of a sample of vegetable soup was found to be 6.5. How is this soup likely to taste?
View AnswerAns. It will be sour in taste.
31. Which bases are called alkalies? Give one example of alkali.
View AnswerAns. Those bases which are soluble in water are called alkalies. E.g., NaOH, KOH
32. Write the name and chemical formula of the product formed by action of chlorine on slaked lime.
View AnswerAns. CaOCl2, Bleaching powder, Calcium oxy-chloride.
33. Write a balanced chemical equation for the reaction between sodium carbonate and hydrochloric acid indicating the physical state of reactants and the products.
View AnswerAns. Na2CO3 (s) + 2HCl (dil) —– > 2NaCl (aq) + CO2 (g) + H2O (l)
34. Name the acid and base that have constituted the salt ammonium nitrate.
View AnswerAns. Acid: HNO3
Base: NH4OH (i.e., Nitric acid, Ammonium hydroxide)
35. Suggest one way to reduce alkaline nature of the soil.
View AnswerAns. Add ammonium nitrate (acidic salt) to neutralize alkaline nature of soil.
36. Oxides of metals are basic while those of non-metals are acidic. Explain.
View AnswerAns. Metal oxides dissolve in water to form base, basic in nature. On the other hand non-metals dissolve in water to form acids, acidic in nature.
37. What is the difference between slaked lime and lime water?
View AnswerAns. The solid Ca(OH)2 is slaked lime whereas clear solution of Ca(OH)2 in water is lime water.
38. Write a balanced chemical equation for the neutralization reaction, mentioning the physical state of reactants and products.
View AnswerAns. NaOH (aq) + HCl (aq) —- > NaCl (aq) + H2O (l)
39. During summer season, a milkman usually adds a very small amount of baking soda to fresh milk. Give one reason.
View AnswerAns. Baking soda is basic in nature; it will not allow milk to turn sour due to the formation of lactic acid.
40. Curd is not kept in copper and brass utensils, why?
View AnswerAns. Curd contains lactic acid which can make poisonous compounds with brass and copper vessels.
41. Fresh milk has pH = 6. When it changes to curd, will its pH value increase or decrease?
View AnswerAns. pH value will decrease when milk changes to curd.
42. What would be the colour of litmus in a solution of sodium carbonate?
View AnswerAns. The red litmus will turn blue in Na2CO3 solution.
43. What is the colour of litmus in a solution of ammonium hydroxide?
View AnswerAns. Red litmus will turn blue green colour in a solution of ammonium hydroxide.
44. On adding dilute hydrochloric acid to copper oxide powder, the solution formed is blue gree. Predict the new compound formed which imparts a blue green colour to the solution.
View AnswerAns. Copper chloride imparts blue green colour to the solution.
45. How does flow of acid rain water into river makes the survival of aquatic life in the river difficult?
View AnswerAns. Acidic water makes aquatic species uncomfortable. Aquatic species are more comfortable in the pH 7 to 7.8.
46. How does the pH change when solution of a base is diluted?
View AnswerAns. When solution of a base is diluted, its pH decreases.
47. Arrange the following in an increasing order or their pH values.
View AnswerNaOH solution, Blood, Lemon juice.
Ans. Lemon Juice < Blood < NaOH solution.
48. At what pH in the mouth is tooth decay faster and why?
View AnswerAns. At pH lower than 5.5, tooth decay becomes faster because calcium phosphate (enamel) reacts with acid and gets corroded.
Two Marks Questions
49. A white chemical compound becomes hard on mixing proper quantity of water. It is also used to maintain joints in fixed position. Name the chemical compound and write its chemical formula. Write the chemical equation to show what happens when water is added to this compound in proper quantity.
View AnswerAns. CaSO4. ½ H2O is the formula of the compound. The name of compound is ‘Plaster of Paris’ (Calcium sulphate hemihydrate)
CaSO4.1/2 H2O + 3/2 H2O —– > CaSO4. 2H2O
Plaster or Paris (Gypsum)
50. Two solutions ‘A’ and ‘B’ have pH value 3.0 and 10.5 respectively. Which of these will turn
a) Blue litmus solution to red.
b) Phenolphthalein from colourless to pink. Justify your answer in each case.
View AnswerAns. a) ‘A’ with pH = 3 will turn blue litmus red because it is acidic in nature.
b) ‘B’ with pH = 10.5 will turn phenolphthalein colourless to pink because ‘B’ is basic in nature.
51. The pH of soil ‘A’ is 7.5, while that of soil ‘B’ is 4.5. Which of the two soils A or B should be treated with powdered chalk to adjust the pH and why?
View AnswerAns. Soil ‘B’ is acidic, therefore it needs to be treated with powdered chalk to adjust its pH because chalk is basic, which will make soil neutral.
52. Write the chemical equation to describe how baking soda is produced on a large scale. Also write the chemical name of the products formed in the reaction.
View AnswerNH3 + H2O + CO2 + NaCl —- > NaHCO3 + NH4Cl
53. What is chlor-alkali process? Write a balanced chemical equation for the reaction involved in this process to justify your answer.
View AnswerAns. When brine solution is electrolyzed we get alkali (NaOH) and chlorine (Cl2) gas, this process is called chlor-alkali process.
2NaCl (aq) + 2H2O (l) —> 2NaOH (aq) + H2 (g) + Cl2 (g)
54. What is meant by the term water of crystallization? How would you show that copper sulphate crystals contains water of crystallization?
View AnswerAns. The molecules of water associated with crytstalline substance are called water of crystallization.
When hydrated copper sulphate is heated its colour changes from blue to dirty white and water droplets are formed.
CuSO4.5H2O ———- > CuSO4 + 5H2O
If we add little water to anhydrous CuSO4, we get blue colour again. It is the presence of molecules of water of crystallization which was lost on heating
CuSO4 + 5H2O —– > CuSO4.5H2O
(Anhydrous)
55. Mention the pH of aqueous solution of the following salts as 7, more than 7, less than 7
KCl, Na2CO3, NH4Cl, NaNO3 (Sodium nitrate)
View AnswerAns. KCl and NaNO3 has pH = 7
Na2CO3 has pH > 7
NH4Cl has pH < 7
56. you have two solutions A and B. The pH of solution ‘A’ is 6 and the pH of solution ‘B’ is 8. Which solution has more hydrogen ion concentration? Which one of this is acidic and which one is basic?
View AnswerAns. ‘A’ has more H+ ion concentration.
‘A’ is acidic while ‘B’ is basic.
57. Give suitable reasons to justify the following statements:
An aqueous solution of sodium chloride is neutral but an aqueous solution of sodium metal is basic.
View AnswerAns. Sodium chloride is made up of a strong base, NaOH and a strong acid HCl. Therefore, its aqueous solution is neutral in nature.
Sodium metal reacts with water to form NaOH (base) and H2 gas
2Na (s) + 2H2O (l) —– > 2NaOH (aq) + H2 (g)
58. What is the action of litmus on
a) dry ammonia gas
b) solution of ammonia gas in water?
View AnswerAns. a) There is no effect of dry litmus on dry ammonia gas
b) Solution of ammonia will turn red litmus blue.
59. State the observations you would make on adding sodium hydroxide to an aqueous solution of
a) ferrous sulphate
b) aluminium chloride
View AnswerAns. a) Green precipitate of Fe(OH)2 will be formed
FeSO4 (aq) + 2NaOH (aq) —– > Fe(OH)2 + Na2SO4 (aq)
(Green ppt)
b) White precipitate of Al(OH)3 will be formed.
AlCl3 + 3NaOH —– > Al(OH)3 (s) + 3NaCl (aq)
(White ppt)
60. 15mL of water and 10mL of sulphuric acid are to be mixed in a beaker.
a) State the method that should be followed with reason.
b) What is this process called?
View AnswerAns. a) Acid should be added to the water slowly with constant cooling because the reaction is highly exothermic
b) This process is called dilution.
61. Name the acid present in the following:
a) Tomato
b) Vinegar
c) Tamarind
View AnswerAns. a) Tomato contains oxalic acid
b) Vinegar contains acetic acid
c) Tamarind contains tartaric acid.
62. Explain how antacid works.
View AnswerAns. Antacids are weakly basic in nature. They neutralize excess of HCl present in our stomach and gives us relief from hyper-acidity.
63. Equal lengths of magnesium ribbon are taken in test tube ‘A’ and ‘B’. Hydrochloric acid (HCl) is added to test tube ‘A’ while acetic acid (CH3COOH) is added to test tube ‘B’. In which test tube, will fizzing occur more vigorously and why?
View AnswerAns. The fizzing will occur more vigorously in test tube ‘A’ because HCL is a strong acid and reacts faster than acetic acid which is a weak acid.
64. State what does pH of solution signify? Three solutions A, B and C have pH values of 6, 2 and 10 respectively. Which one of these solutions is highly acidic? Which solution will turn red litmus blue?
View AnswerAns. pH of solution signifies the nature of the solution i.e., it is weakly acidic, strongly acidic, neutral weakly basic, strongly basic.
‘B with pH = 2 is strongly acidic
‘C’ with pH = 10 will turn red litmus blue.
65. Define an acid and a base. Name one weak acid and one strong acid.
View AnswerAns. Acid is a substance which gives H+ ions in an aqueous solution.
Base is substance which gives OH– ions in the aqueous solution.
CH3COOH is a weak acid, H2SO4is a strong acid.
66. What is universal indicator? State the purpose for which this indicator is used.
View AnswerAns. Universal indicator is a mixture of a number of indicators. It is used to determine pH of a solution.
67. Name the natural sources of each of the following acid.
a) Citrix acid
b) Oxalic acid
c) Lactic acid
d) Tartaric acid
View AnswerAns. a) Citrix acid – Lemon, Orange
b) Oxalic acid – Tomato, Guava
c) Lactic acid – Curd, Sour milk
d) Tartaric acid – Tamarind
68. Explain why sodium hydroxide solution cannot be kept in aluminium containers? Write the equation for the reaction that may take place for the same.
View AnswerAns. It is because ‘Al’ reacts with NaOH to form sodium meta-aluminate and hydrogen gas.
2Al + 2NaOH + 2H2O —- > NaAlO2 + 3H2
69. A student detected the pH of four unknown solutions A, B, C and D as follows: 11, 5, 7 and 2. Predict the nature of these solutions.
View AnswerAns. pH = 11 is basic
pH = 5 is acidic
pH = 7 is neutral
pH = 2 is strongly acidic
70. Give two uses of baking soda and washing soda each.
View AnswerAns. Use of baking soda:
a) It is used in making of bread, biscuits, cakes
b) It is used as an antacid.
Use of Washing soda:
a) It is used as a cleansing agent
b) It is used to remove hardness of water.
71. A compound ‘X’ of sodium is commonly used for making crispy pakoras. It is also used for curing acidity in the stomach. Identify ‘X’. Write the formula and its chemical name. State the reaction which takes place when it is heated.
View AnswerAns. ‘X’ is NaHCO3, sodium hydrogen carbonate. It is used in cooking and for curing acidity in stomach.
2NaHCO3 —– > Na2CO3 + CO2 + H2O
72. Crystals of a substance changes their colour on heating in a closed vessel but regained it after sometime, when they are allowed to cool down.
a) Name one such substance
b) Explain the phenomenon involved.
View AnswerAns. a) CuSO4.5H2O (Hydrated copper sulphate)
b) CuSO4.5H2O —– > CuSO4 + 5H2O
(Blue) (Dirty white)
The colour changes due to the loss of molecules of water of crystallization. Colour is regained by absorbing water molecules from atmosphere containing water vapours.
73 (a) Write the name given to the bases that are highly soluble in water. Give an example.
b) Why does bee sting causes pain and irritation? Rubbing of baking soda on the sting area gives relief. How?
View AnswerAns. a) Highly soluble bases are called alkalies e.g., KOH
b) Bee sting contains HCOOH, formic acid which causes irritation. Baking soda (basic) neutralizes HCOOH, therefore it gives relief from pain on rubbing it on sting area.
74. The pH of the mouth of a person is lower than 5.5. What changes will occur in his mouth? How these changes can be controlled? Write any two measures.
View AnswerAns. Acid will be formed in the mouth which causes tooth decay.
a) Wash your mouth with water after every meal.
b) Brush your teeth after meal. Toothpastes are basic in nature and it will neutralize the acid formed in mouth.
75. What is a neutralization reaction? Give one example.
View AnswerAns. The reaction in which acid reacts with a base to form salt and water is called neutralization reaction e.g.,
KOH (aq) + HNO3 (aq) —- > KNO3 (aq) + H2O (l)
76. Write the chemical name of Plaster of Paris. Write a chemical equation to show the reaction between Plaster of Paris and water.
View AnswerAns. CaSO4. 1/2H2O (Calcium sulphate hemihydrate)
CaSO4.1/2 H2O + 3/2H2O —– > CaSO4. 2H2O
77. State in brief the preparation of washing soda from baking soda. Write balanced chemical equation of the reaction involved.
View AnswerAns. When sodium hydrogen carbonate (Baking soda) is heated, sodium carbonate is formed which on crystallization forms washing soda.
2NaHCO3 ——- > Na2CO3 + CO2 + H2O
(Baking soda)
Na2CO3 + 10H2O ——- > Na2CO3.10H2O
(Washing soda)
78. What is colour of FeSO4.7H2O crystals? How does this colour change upon heating? Give a balanced chemical equation for the change.
View AnswerAns. FeSO4.7H2O is pale green in colour. It becomes dirty white on heating.
FeSO4.7H2O ——- > FeSO4 + 7H2O
If it is heated strongly Fe2O3 and SO2, SO3 gases will be formed.
2FeSO4 ——- > Fe2O3 + SO2 + SO3
79. Classify the following salts into acidic, basic and neutral salts:
Potassium sulphate, ammonium chloride, sodium carbonate, sodium chloride.
View AnswerAns. Acidic: Ammonium chloride
Basic: Sodium carbonate
Neutral: Potassium sulphate, sodium chloride.
80. A student dropped few pieces of marble in dilute HCl contained in a test tube. The evolved gas was passed through lime water.
a) What change would be observed in lime water?
b) Write a balanced chemical equation for the above change.
View AnswerAns. a) Lime water will turn milky.
b) Ca(OH)2 (aq) + CO2 (g) —– > CaCO3 (s) + H2O (l)
81. A white powder is added while baking breads and while making cakes to make them soft and fluffy. What is the name of that powder? What are the main ingredients in it? What are the functions of each ingredient?
View AnswerAns. The powder is baking powder. It consists of sodium hydrogen carbonate and tartaric acid.
NaHCO3 gives CO2 on heating which makes the bread cake soft and fluffy. Tartaric acid neutralizes Na2CO3 which is bitter in taste.
82. HCl and HNO3 show acidic characteristics in aqueous solution while alcohol and glucose solutions do not. Give reasons.
View AnswerAns. HCl and HNO3 form H+ or H3O+ ions in aqueous solution whereas alcohol and glucose do not dissociate into ions.
HCl + H2O —— > H3O+ + Cl–
HNO3 + H2O —– > H3O+ + NO3–
83. What is bleaching powder chemically? Give a reaction for its preparation. State one of its use.
View AnswerAns. Bleaching powder is chemically CaOCl2, calcium oxychloride
Ca(OH)2 + Cl2 —- > CaOCl2 + H2O
It is used as a disinfectant i.e., it makes water fit for drinking.
84. What are olfactory indicators? Dry HCl gas does not change the colour of dry blue litmus. Give reason.
View AnswerAns. Olfactory indicators: they give different smell in acids and bases.
Dry HCl (g) does not form ions, so there is no effect on litmus.
85. Answer the following:
a) Why is Plaster of Paris written as CaSO4.1/2 H2O? How is it possible to have a half water molecule attached with CaSO4?
b) Why is sodium hydrogen carbonate an essential ingredient in antacids?
View AnswerAns. It has one molecule of water associated with 2 molecules of CaSO4. Water molecules are present as water of crystallization.
b) It is a mild base and it can neutralize hyper acidity without harming our body.
86. What happens when chlorine is passed over slaked lime at 313 K? Write a chemical equation of the reaction involved and state two uses of product.
View AnswerAns. Bleaching powder, CaOCl2 is formed:
CaOCl2 + Cl2 ——- > CaOCl2 + H2O
a) It is used as an oxidizing agent.
b) It is used as a disinfectant.
87. What is meant by ‘water of crystallisation’ of a substance? Describe an activity to show that blue copper sulphate crystals contains water of crystallization.
View AnswerAns. the molecules of water associated with crystalline substance are called ‘water of crystallisation’.
CuSO4.5H2O ——- > CuSO4 + 5H2O
When hydrated copper sulphate is heated its colour changes from blue to dirty white and water droplets are formed. If we add little quantity of water to anhydrous CuSO4, we get blue colour again. It is those presence of molecules water of crystallisation which was lost on heating.
Activity: To study the effect of heat on hydrated crystalline salts.
i) Take 2 g of CuSO4.5H2O in a test tube.
ii) Observe the initial colour of the salt.
iii) Heat the test tube at top of burner carefully as shown in the diagram.
iv) Record your observations.
v) Cool the crystals and add few drops of water.
vi) Record your observations again.
Observations: Blue colour of CuSO4.5H2O is changed to dirty white anhydrous CuSO4 and water droplets were formed. On adding water, blue colour of salt was restored.
Conclusion: CuSO4.5H2O is a hydrated salt which loses water of crystallisation, which on heating becomes dirty white and regains its colour when it comes in contact with water.
Chemical reactions involved:
CuSO4.5H2O (s) —— > CuSO4 (s) + 5H2O (l)
(Blue) (Dirty white)
CuSO4 (s) + 5H2O (l) —— > CuSO4.5H2O
(Blue)
88. Write the chemical formulae of washing soda and baking soda. Which one of these two is an ingredient of antacids? How does it provide relief in stomach ache?
View AnswerAns. Na2CO3.10H2O is washing soda, NaHCO3 is baking soda. NaHCO3 is an ingredient of antacid. It neutralizes hyper acidity in stomach and gives relief.
89. What is baking powder? How does it make the cake soft and spongy?
View AnswerAns. Baking powder is made up of NaHCO3 and tartaric acid. NaHCO3, on heating gives CO2 which makes the cake soft and spongy.
Three Marks Questions
90. 2mL of sodium hydroxide solution is added to a few pieces of granulated zinc metal taken in a test tube. When the contents are warmed, a gas evolves which is bubbled through a soap solution before testing. Write the equation for the chemical reaction involved and the test to detect the gas. Name the gas which will be evolved when the same metal reacts with dilute solution of a strong acid.
View AnswerAns. Zn (s) + 2NaOH —— > Na2ZnO2 + H2
Test: Bring a burning splinter near the gas. If it burns with ‘pop’ sound, the gas liberated is hydrogen.
Zn + H2SO4 (dil) —– > ZnSO4 (aq) + H2
Hydrogen gas will be evolved by reaction of the same metal with dilute H2SO4, strong acid.
91. The pH of a salt which is used to make tasty and crispy pakoras is 14. Identify the same and write a chemical equation for its formation. List its two uses.
View AnswerAns. The salt is NaHCO3, sodium hydrogen carbonate.
NH3 (g) + CO2 (g) + NaCl (g) + H2O (l) —–> NaHCO3 (s) + NH4Cl
Uses:
a) It is used as an antacid
b) It is used in soda-acid fire extinguishers.
No salt has pH = 14. NaHCO3 has pH = 8.4
92. (a) Why does aqueous solution of an acid conduct electricity?
(b) How does the concentration of H3O+ ions change when a solution of an acid is diluted?
(c) Which one has a higher pH, a concentrated or a dilute solution of hydrochloric acid?
(d) What would be the gas evolved on adding dilute hydrochloric acid to
(i) Solid sodium carbonate placed in a test tube?
(ii) Zinc metal in a test tube?
View AnswerAns. a) It contains ions which carry current.
b) H3O+ ions will decrease when it is diluted
c) Dilute solution has higher pH than concentrated solution.
d. (i) CO2 gas will be formed.
Na2CO3 + 2HCl —– > 2NaCl + H2O + CO2
(ii) Hydrogen gas will be formed.
Zn + 2HCl —- > ZnCl2 + H2
93. pH as a great importance in our daily life. Explain by giving three examples.
View AnswerAns. a) pH of our stomach is 2.0 and it is needed for the digestion of proteins in our body.
b) Blood has pH = 7.36 to 7.42 which must be maintained for proper health.
c) pH of soil is determined and suitable chemicals are added so as to make it suitable for growth of crops.
94. Answer the following questions:
a) State the colour of phenolphthalein in soap solution.
b) Name the by-product of chlor-alkali process which is used for the manufacture of bleaching powder.
c) Name one indicator which specifies the various levels of H+ ion concentration.
View AnswerAns. a) Phenolphthalein will turn pink in soap solution.
b) Chlorine is the by-product of chlor-alkali process which is used in the manufacture of bleaching powder.
c) Universal indicator specifies the various levels of H+ ion concentration.
95. a) Define a universal indicator. Mention its one use.
b) Solution ‘A’ gives pink colour when a drop of phenolphthalein indicator is added to it. Solution ‘B’ gives a red colour when a drop of methyl orange is added to it. What type of solutions are ‘A’ and ‘B’ and which of these will have higher pH?
c) Name one salt whose solution has pH greater than 7 and one salt with pH less than 7.
View AnswerAns. a) Universal indicator is mixture of indicators used to find pH of solution. It is used to measure levels of H+ ion concentration.
b) ‘A’ is basic in nature, ‘B’ is acidic in nature. ‘A’ will have higher pH than ‘B’. it should be greater than 7.
c) Na2CO3 is the salt whose pH is more than 7, CuSO4 is the salt whose pH is less than 7.
96. a) Define pH scale. Draw a figure showing variation of pH with change in concentration of H+ (aq) and OH– (aq) ions.
b) Mention the pH of acidic, basic and neutral solutions respectively.
View AnswerAns. a) pH scale is a scale which is used for measuring hydrogen ion concentration in a solution.
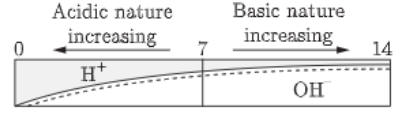
b) pH < 7 for acidic solution, pH > 7 for basic solution, pH = 7 for neutral solution.
97. a) Define olfactory indicators. Name two substances which can be used as olfactory indicators.
b) Choose strong acids from the following:
CH3COOH, H2SO4, H2CO3, HNO3
View AnswerAns. a) Olfactory indicators: They give different smell in acidic and basic medium e.g. onion, clove, vanilla.
b) HNO3 and H2SO4 are strong acids among the given acids.
98. Explain the action of dilute hydrochloric acid on the following with suitable chemical equations.
a) Magnesium ribbon
b) Sodium hydroxide
c) Crushed egg shells
View AnswerAns. a) Mg (s) + 2HCl (dil) —- > MgCl2 (aq) + H2 (g)
b) NaOH (aq) + HCl (dil) —– > NaCl (aq) + H2O (l)
c) CaCO3 (s) + 2HCl (dil) —– > CaCl2 (g) + CO2 (g) + H2O (l)
99. (a) The blue colour of crystals of a substance on heating in a closed test tube gets changed but the colour was regained after sometime on cooling. Name that substance and write its chemical formula. Explain the phenomenon involved.
b) Write name and chemical formulae of two such compounds whose one unit is associated with 10 and 2 molecules respectively.
View AnswerAns. a) Hydrated copper sulphate, CuSO4.5H2O is the name and chemical formula of that substance. It loses water of crystallisation on heating and regains these molecules of water on exposure to the atmosphere.
CuSO4.5H2O —— > CuSO4 + 5H2O
(Blue) (Dirty White)
CuSO4 (s) + 5H2O (l) —– > CuSO4.5H2O
b) Na2CO3.10H2O, washing soda (Sodium carbonate decahydrate) has 10 molecules of water of crystallisation. CaSO4.2H2O, gypsum, chemically calcium sulphate dehydrate has 2 molecules of water of crystallisation.
100. you are provided with magnesium ribbon and Sulphur powder. Explain with the help of activity that metal oxides are basic and oxides of non-metals are acidic in nature.
View AnswerAns. Burn magnesium ribbon with the help of tongs to form white ash. Dissolve the ash in hot water. Add red litmus which turns blue, showing that MgO is a basic oxide.
2Mg (s) + O2(g) —- > 2MgO (s)
MgO + H2O (hot) —- > Mg(OH)2 (aq)
Heat Sulphur taken in a iron spatula and pass the gas through water. Add blue litmus into it. It will turn red showing SO2 is an acidic oxide.
S + O2 —- > SO2
SO2 + H2O —– > H2SO3 (Sulphurous acid)
101. List two difference between acids and bases on the basis of chemical properties.
View AnswerAns.
| Acids | Bases |
| 1. Acids turn blue litmus red | 1. Bases turn red litmus blue |
| 2. Acids liberate CO2 with metal carbonates and hydrogen carbonates | 2. Bases do not react with metal carbonates and hydrogen carbonates. |
102. A Substance ‘X’ is used as antacid reacts with hydrochloric acid to produce a gas Y which is used in fire extinguishers:
a) Name the substance X and Y.
b) Write a balanced equation of the reaction between X and hydrochloric acid.
View AnswerAns. a) ‘X’ is NaHCO3 (Sodium hydrogen carbonate). ‘Y’ is CO2 gas, which is used in fire extinguishers.
b) NaHCO3 (s) + HCl (aq) ——- > NaCl (aq) + H2O (l) + CO2 (g)